Building Medical Device Innovations Together
Case Studies
Our approach allows us to provide services to meet several objectives in parallel while also identifying new solutions, or “bonus objectives,” that give our clients a unique competitive advantage.
View a sample of case studies below to see how Telos has helped our partners achieve success through our unique and integrated approach.
Proven Results Matter: Client Case Studies
Typically, our clients come to Telos with one objective in mind. We go above and beyond to help identify and seamlessly address other areas where we can provide value that may not have initially been on the client’s radar. This translates to trust in Telos to always have the company’s best interest at heart.
The result? An unmatched competitive advantage for our clients and a blueprint for continued growth and success.
Focus Objective Clinician Adoption
Client Type:
Spine device manufacturer
Original Need:
“We need to gather clinical data through a retrospective study to meet the needs of publication for market access and clinician adoption. We also need our initial CE Mark.”
Where Telos Helped:
Clinical research services, evidence services, regulatory and quality services.
Bonus Outcome due to Integration:
Increased clinician adoption
Focus Objective Reimbursement
Client Type:
Discectomy device manufacturer
Original Need:
“We need help organizing our unpublished clinical data to prepare for commercialization.”
Where Telos Helped:
Evidence services, clinical research, regulatory and quality
Bonus Outcome due to Integration:
Increased clinician adoption by addressing a reimbursement issue where a code was not yet in place for the device.
Four Steps to the
“Ultimate Objective”
As your partner, Telos invests time and effort from the onset of connection to understand your ultimate business objectives. This approach helps us to prescribe and provide the right services for success. Telos means “ultimate objective” – let us help you reach yours.
Define Your Goals
Telos works with you to clearly understand your business’ goals. We ask “Why” instead of “How.”
Identify Services & Plan
Our team identifies service areas where we can partner to create a plan unique to your business.
Deliver Solutions
We’ll work with you through each phase to accomplish the milestones identified in the plan.
Repeat as Business Scales
Because we see each client as a true partner, we remain ready to help your business scale as new needs arise.
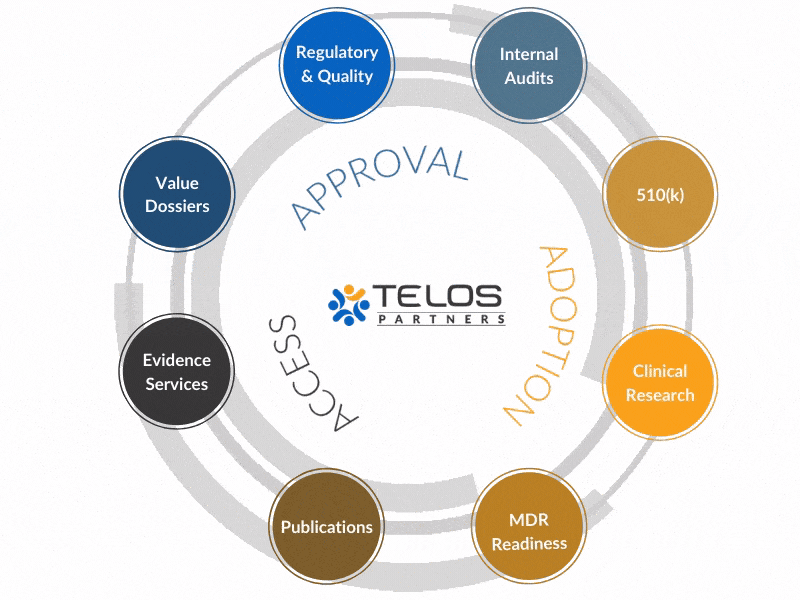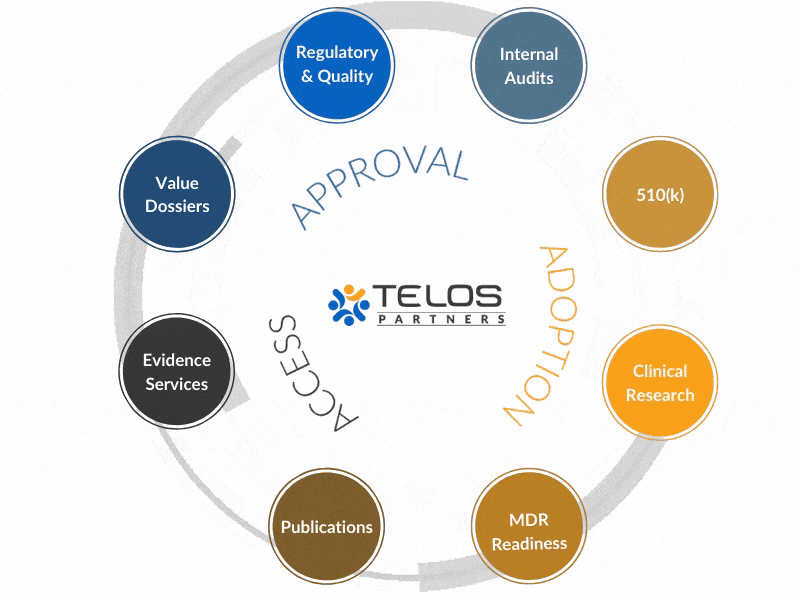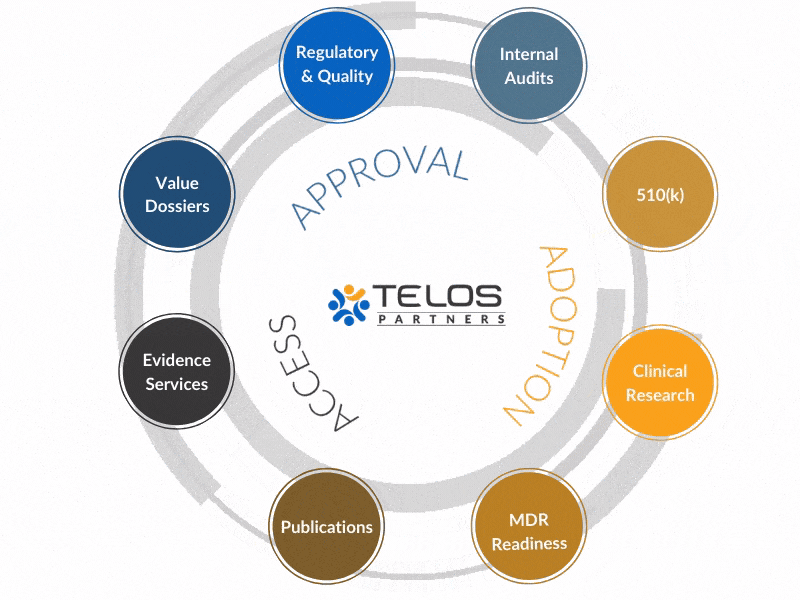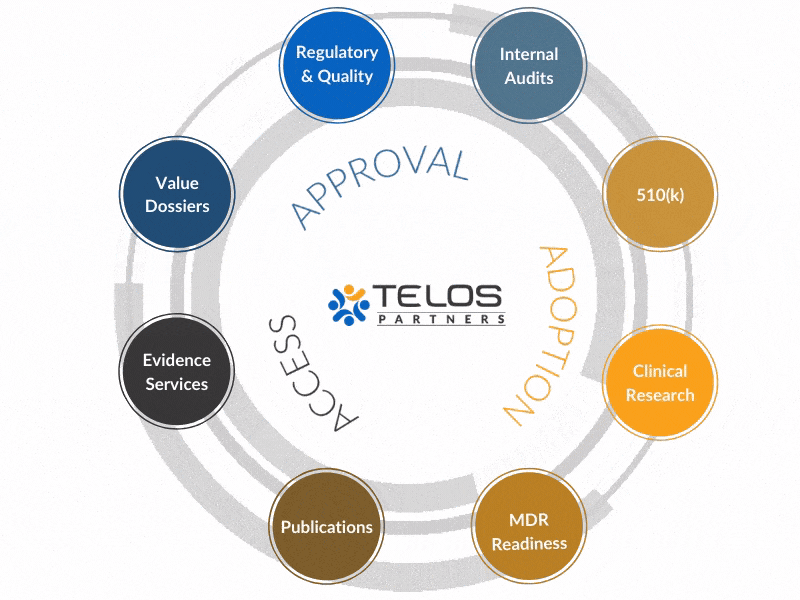


Why An Integrated Approach?
Our evidence, CRO, regulatory, and quality services operate in a unique, integrated fashion to achieve your ultimate goals by circling-up around each objective.
Instead of providing services, we work to provide intrinsic value to your success.