Building Medical Device Innovations Together
Solution:
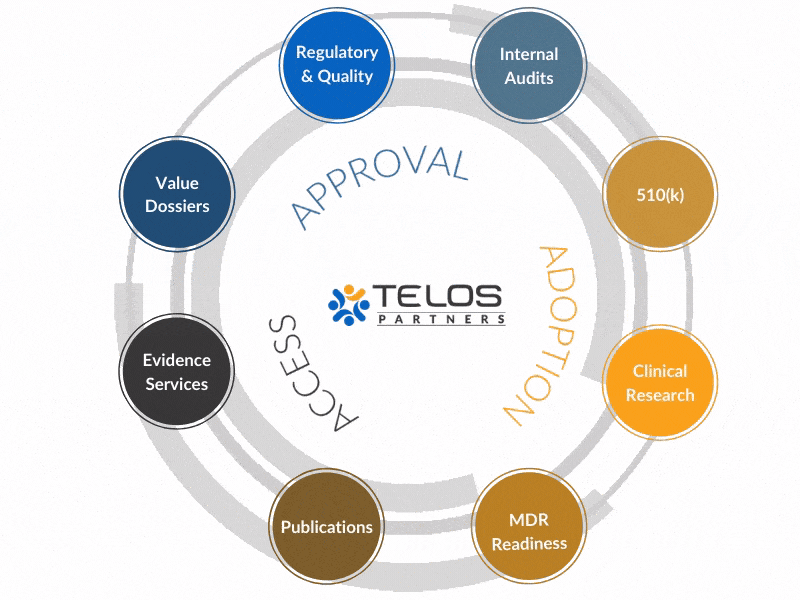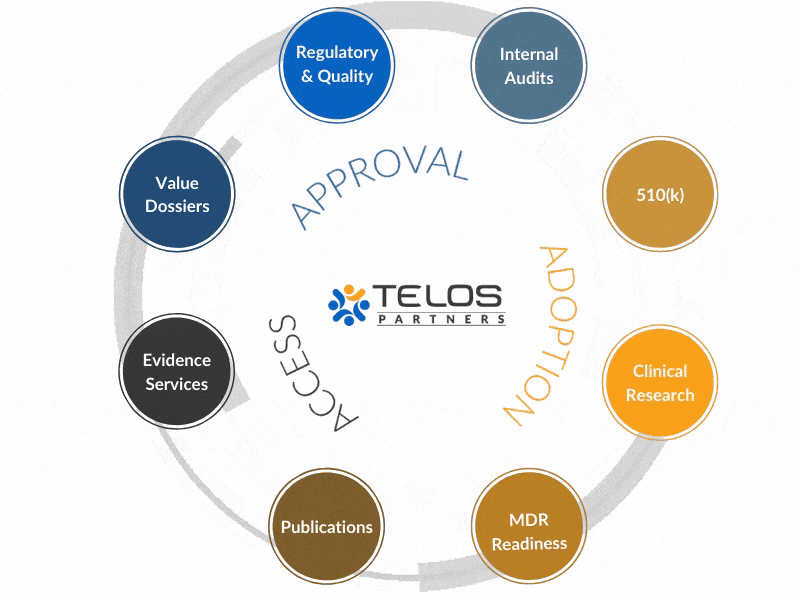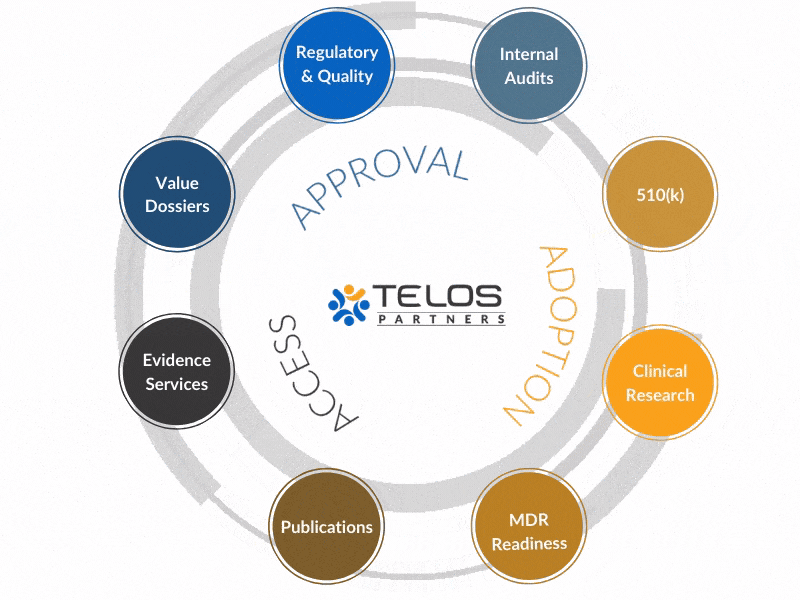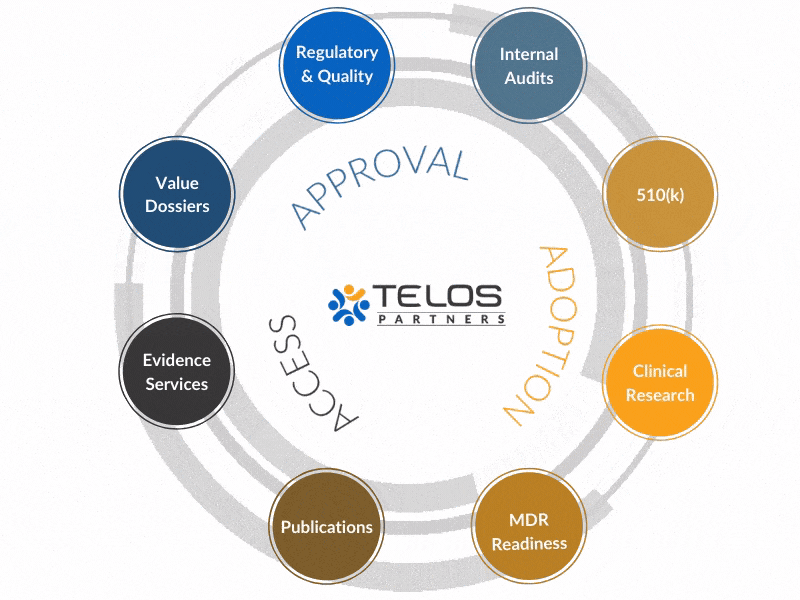
Regulatory & Quality
The regulatory space can seem confusing and complex. That’s where we come in. Our friendly team helps to demystify the complexities of regulatory pathways and quality systems. Working together, you’ll be guided by a concrete strategy backed by confident execution of deliverables.
Regulatory & Quality Services
Example services that our regulatory and quality experts can
assist you with include:
- Regulatory landscape and strategy
- Internal audits
- Clinical evaluation reports (CERs)
- Q-submissions and FDA interaction
- Clinical study IDE, IND applications
- 510(k), De Novo, PMA, BLA
- Breakthrough or RMAT designation
- Quality systems, SOPs, MDSAP
- MDR Gap analysis and strategy
- PMS and PMCF procedures
- CE Mark
- Technical files
Telos strives to be more than just “regulatory and quality specialists.” We put ourselves in your shoes to ensure our services are in lockstep with your goals as if Telos is an extension of your business and a part of your team.
Our Unique Approach Combined with Years of Expertise Provides Our Clients With Solutions They Can Trust and Scale
Telos by the Numbers
Years of Regulatory & Quality Experience
Our R&Q Services Integrate with Many Telos Solutions to Provide the Best Possible Solutions and Service for Our Clients
Four Steps to the
“Ultimate Objective”
As your partner, Telos invests time and effort from the onset of connection to understand your ultimate business objectives. This approach helps us to prescribe and provide the right services for success. Telos means “ultimate objective” – let us help you reach yours.
Define Your Goals
Telos works with you to clearly understand your business’ goals. We ask “Why” instead of “How.”
Identify Services & Plan
Our team identifies service areas where we can partner to create a plan unique to your business.
Deliver Solutions
We’ll work with you through each phase to accomplish the milestones identified in the plan.
Repeat as Business Scales
Because we see each client as a true partner, we remain ready to help your business scale as new needs arise.
